Abstract
Treatment of acute myeloid leukemia (AML) remains challenging, particularly for patients unfit for intensive chemotherapy. Venetoclax, a BCL2 inhibitor, has significantly improved treatment outcomes for these AML patients unfit for induction chemotherapy. Our group has pioneered the production of natural killer (NK) cells from human induced pluripotent stem cells (iPSCs). iPSC-derived NK cells effectively kill AML cells, similar to NK cells isolated from peripheral blood or cord blood. iPSCs provide an advantage of a stable platform for gene modifications and previous studies demonstrate we can engineer iPSCs to express or delete genes of interest to derive genetically modified iPSC-NK cells with improved anti-tumor activity. Based on the characterization of the BCL2 G101V mutation that provides resistance to the BCL2 inhibitor venetoclax in chronic lymphocytic leukemia, we investigated the effect of homozygous BCL2 G101V knock-in in iPSC-derived NK cells to test the hypothesis that these resistant cells could be combined with venetoclax to improve NK cell-mediated killing of AML cells.
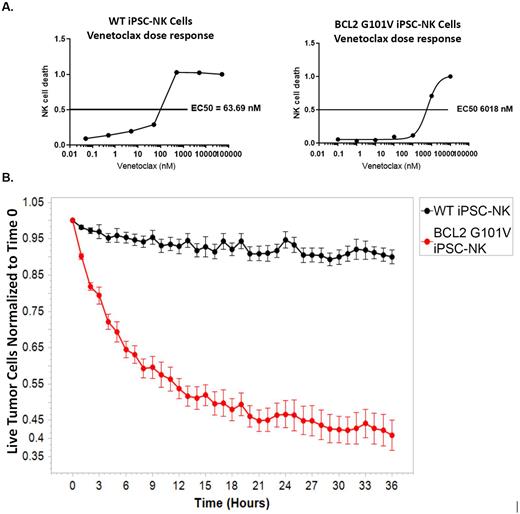
To generate iPSC-derived NK cells with an improved ability to tolerate exposure to venetoclax, we employed CRISPR-Cas9 technology to knock-in the BCL2 G101V mutation (BCL2G101V) in iPSCs. iPSCs homozygous for BCL2G101V were selected and differentiated to NK cells. BCL2G101V iPSC-NK cells were 94-fold more resistant to venetoclax compared to wildtype iPSC NK cells (Panel A). Analysis of cell surface proteins showed that both sets of iPSC-NK cells had a typical NK cell phenotype with no differences in expression of receptors analyzed. There was no difference seen in cytotoxicity against K562 tumor cells between BCL2G101V iPSC-NK cells and WT-iPSC NK cells.
Additional functional analyses demonstrated that activity of the BCL2G101V iPSC NK cells was preserved upon exposure to venetoclax. CD107a expression on WT iPSC-NK cells stimulated by MOLM13 AML tumors cells was reduced nearly 3-fold upon addition of venetoclax. In contrast, activation of BCL2G101V iPSC NK cells was not significantly affected by addition of venetoclax. This resistance to venetoclax was confirmed in longer term 36-hour Incucyte cytotoxicity assays where BCL2G101V iPSC-NK cells killed more than 10-fold the number of tumor cells than the WT-iPSC-NK cells (Panel B).
Intriguingly, BCL2G101V iPSC NK cells also demonstrated improved cytotoxicity against MOLM13 cells resistant to venetoclax. Further analyses into the mechanism of this property of BCL2G101V iPSC NK cells is in progress. In vivo studies testing the BCL2 G101V iPSC NK cells in combination with venetoclax against WT and venetoclax-resistant MOLM13 of iPSC-NK cells in mouse xenograft-models are also ongoing. Together our results demonstrate that iPSC-NK cells can be engineered to generate venetoclax-resistant cells to be used in combination with venetoclax therapy to improve treatment of AML. Furthermore, this work demonstrates that novel drug resistance mechanisms can be developed by genome engineering of iPSC-derived NK cells as a new strategy to produce improved cell products for "off-the-shelf" therapy.
Disclosures
Kaufman:Qihan Biotech: Consultancy; VisiCell: Consultancy; Shoreline Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.